Rapid Prototyping for Medical Devices: Speed & Readiness

Benefits of Rapid Prototyping in Medical Devices

1. Iterate at the speed of thought
2. Catch mistakes before they get expensive

3. Accelerate clinical input & user validation
4. Strengthen your FDA submission package
5. Align everyone—fast
Common Applications in the Medical Field

1. Surgical Planning Models
2. Custom Surgical Guides
3. Diagnostic Device Housings
4. Implant Prototypes for Form & Fit Testing
5. Educational & Demonstration Tools
Rapid Prototyping vs Traditional Manufacturing
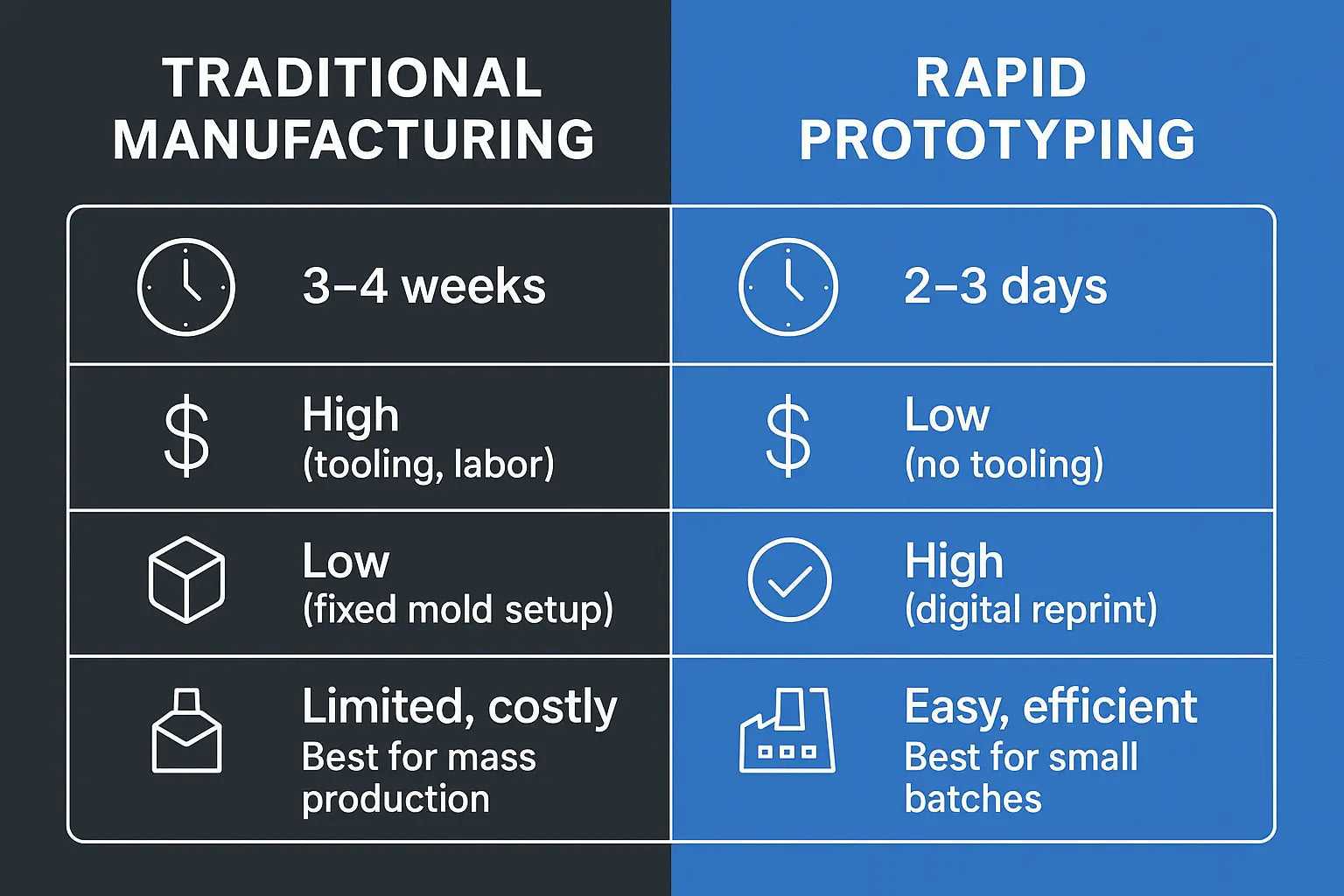
Time-to-Prototype
Cost per Iteration
Design Flexibility
Suitable Production Volume
Risk Mitigation
Case Studies from the Medical Industry
Case 1: Philips-From 12 Weeks to 2
Case 2: Medtronic-Early Feedback Saves $50K
Case 3: GE HealthCare-Speeding Up Regulatory Prep
Regulatory & Testing Considerations
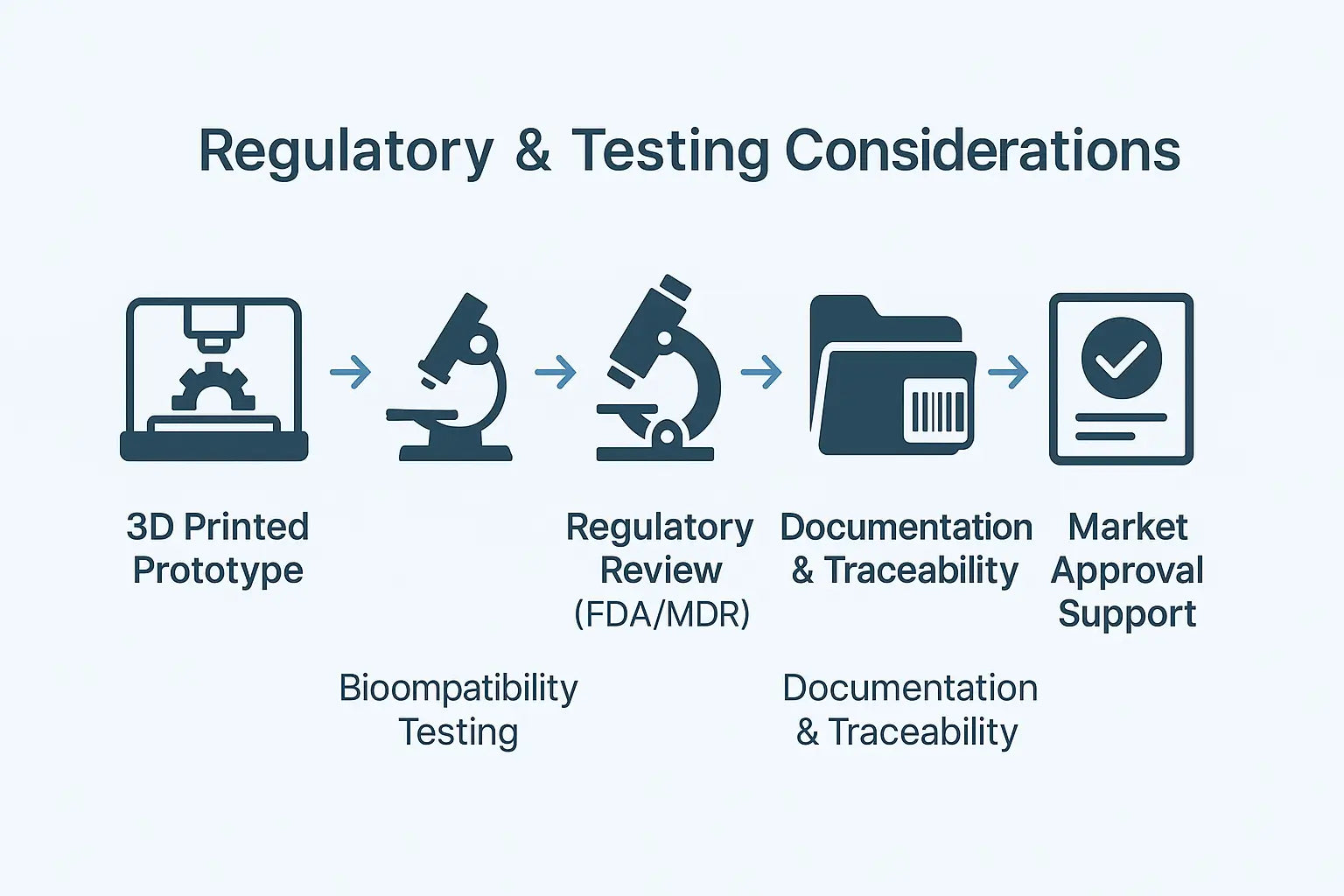
Early Usability Testing
Design Validation Before Freeze
Biocompatibility Planning & Risk Reduction
Documentation for Traceability
How to Choose Medical Prototyping Partner

